DSRP in Non-Neural Life Forms and Nonliving Compounds
This blog has been partially adapted from A Literature Review of the Universal and Atomic Elements of Complex Cognition.
Like a pack of wolves, tiny bacteria band together to hunt their evasive prey. In the soil underfoot, a nematode (“round worm”) avoids a deadly chemical. A cell runs toward oxygen to take part in photosynthesis and make the planet habitable by humans[1]. Brightly colored water droplets animate and chase each other across a glass landscape[2]. These are a few of the amazing microscopic dramas that occur just out of range of sight. Microbes and even non-living molecules use DSRP to sense and respond to their environment in a process called chemotaxis.
Chemotaxis is a relatively well-known phenomenon discovered in 1881 by Theodor Wilhelm Engelmann[3] among others. Chemotaxis occurs when receptors on the bacterium distinguish between specific chemical compounds that prompt the bacterium to respond (or in the case of non living a chemotaxis-like process through energy gradients, etc.). There have been over 600 types of receptors identified, and some bacteria express over 130 simultaneously.
Merriam-Webster defines Chemotaxis as "orientation or movement of an organism or cell in relation to chemical agents." Bacteria can respond to numerous stimuli in their environments from the concentrations of nutrients, toxins, oxygen levels, or pH, to osmolarity (the concentration of a solution), to the intensity and wavelength of light[4]. A common response is movement, but chemical secretion and even gene expression[5] are also frequent responses. Chemotaxis benefits the cell, and the organism as a whole. For example, chemotaxis is at play in fetal development of the nervous system, tissue maintenance, tissue restoration and wound healing[6], as well as other processes such as pathogenicity (disease causing), symbiotic interactions, and the creation of biofilms. And, chemotaxis is critically important for the proper functioning of the immune system[7].
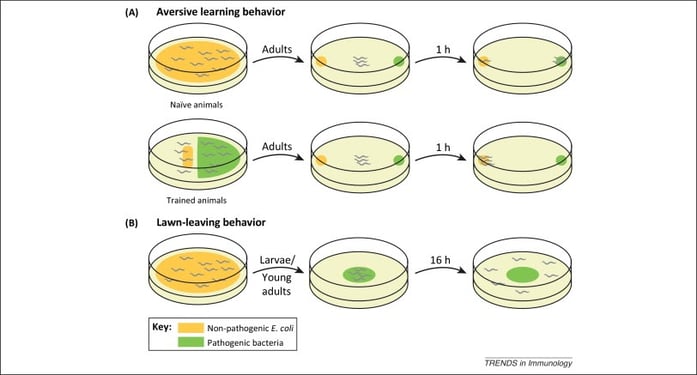
Figure 1: Caenorhabditis elegans avoids Serratia marcescens through chemosensation[8]
In Figure 1, we see that Caenorhabditis elegans (C. elegans), a common nematode present in soil depicted as squiggly lines in the picture, systematically avoids the poisonous bacterium, and deadly pathogen (shown in green), Serratia marcescens[9] via chemotaxis. Experiments “combining bacterial genetics and nematode genetics” show that C. elegans specifically avoids certain strains of the pathogen (Serratia) by producing an organic compound (cyclic lipodepsipentapeptide serrawettin W2).[10] What this nematode demonstrates is that it maintains a perspective that can distinguish a chemical from the rest of its environment, then draw a relationship to the dangerous pathogen, and subsequently avoid it. It also demonstrates learning, as it alters its olfactory sensors after exposure to the pathogen, thereby avoiding contact in the future.[11]
Figure 2 shows a microbe named M. xanthus, that hunts in packs by picking up on a chemical secretion emitted by its prey[12]. By measuring the concentration of this secretion, M. xanthus knows the distance between itself and the prey. Alternatively, the prey has its own chemotaxic perspective, as they can also distinguish between their environment and the chemical secretions of the predator, resulting in a continuous cat and mouse game. May the best Distinguisher win! Amazingly, M. xanthus uses a group-form of chemotaxis to determine: (1) if there are enough bacterium to hunt, (2) where the prey is, and, (3) how much chemical to release both individually and collectively in order to break down their prey so that they can absorb the energy they need to grow[13].

Figure 2: Group and solitary-based predation by M. xanthus. (C) shows the “three traits of group-mediated predation: colony invasion (top), rippling wave structures (middle), and fruiting bodies (bottom)” [14][15]
Even non-living material such as water droplets are able to use a form of chemotaxis[16]. A Stanford biology and physics lab accidentally discovered odd behavior in droplets of water with food coloring in them. The droplets appeared to “sense” each other and would move in peculiar ways (See video below). Governed by molecular physics, these droplets behave in a chemotaxis-esque way, exhibiting behavior where they “choose” similarly colored droplets (Figure 3) or or "attract" or “chase” other droplets (Figure 4). This occurs through various methods. For example, it occurs when droplets chemosense high or low energy and move accordingly across a gradient of surface energy.

Figure 3: Droplet "choosing" behavior
 Figure 4: Droplet "chasing" and "attracting" behavior
Figure 4: Droplet "chasing" and "attracting" behavior
From non-neural living cells and bacteria to nonliving molecules and compounds, chemotaxis—the perspectival process of distinguishing among things in the environment and then relating that awareness to the appropriate action—is the norm. Indeed, there is even part-whole discernment, as the bacterium distinguish the parts of their environment that can either hurt or help them, and change their behavior accordingly. Chemotaxis ensures the survival of these organisms as their environment is constantly changing, and they must quickly respond to it, or cease to exist. Chemotaxis is one of many research examples[17] of how DSRP is found not only in the mind, but also in nature. It therefore provides insight into how DSRP provides a unique bridge between the physical and natural sciences and the cognitive and social sciences.
For more on DSRP read the book, Systems Thinking Made Simple
Citations
[1] Drews, Gerhart. 2005. “Contributions of Theodor Wilhelm Engelmann on Phototaxis, Chemotaxis, and Photosynthesis.” Photosynthesis Research 83 (1): 25–34.
[2] Cira NJ, Benusiglio A, Prakash M. Vapour-mediated sensing and motility in two-component droplets. Nature. 2015;519: 446–450. doi:10.1038/nature14272
[3] Drews, Gerhart. 2005. “Contributions of Theodor Wilhelm Engelmann on Phototaxis, Chemotaxis, and Photosynthesis.” Photosynthesis Research 83 (1): 25–34.
[4] Wadhams, George H., and Judith P. Armitage. 2004. “Making Sense of It All: Bacterial Chemotaxis.” Nature Reviews. Molecular Cell Biology 5 (12): 1024–37.
[5] Wadhams, George H., and Judith P. Armitage. 2004. “Making Sense of It All: Bacterial Chemotaxis.” Nature Reviews. Molecular Cell Biology 5 (12): 1024–37.
[6] Janetopoulos, Christopher, and Richard A. Firtel. 2008. “Directional Sensing during Chemotaxis.” FEBS Letters 582 (14): 2075–85.
[7] Janetopoulos, Christopher, and Richard A. Firtel. 2008. “Directional Sensing during Chemotaxis.” FEBS Letters 582 (14): 2075–85.
[8] Meisel JD, Kim DH. Behavioral avoidance of pathogenic bacteria by Caenorhabditis elegans. Trends Immunol. 2014;35: 465–470. doi:10.1016/j.it.2014.08.008
[9] Meisel JD, Kim DH. Behavioral avoidance of pathogenic bacteria by Caenorhabditis elegans. Trends Immunol. 2014;35: 465–470. doi:10.1016/j.it.2014.08.008
[10] Citation: Pradel, Elizabeth, Yun Zhang, Nathalie Pujol, Tohey Matsuyama, Cornelia I. Bargmann, and Jonathan J. Ewbank. 2007. “Detection and Avoidance of a Natural Product from the Pathogenic Bacterium Serratia Marcescens by Caenorhabditis Elegans.” Proceedings of the National Academy of Sciences of the United States of America 104 (7): 2295–2300.
[11] Citation: Pradel, Elizabeth, Yun Zhang, Nathalie Pujol, Tohey Matsuyama, Cornelia I. Bargmann, and Jonathan J. Ewbank. 2007. “Detection and Avoidance of a Natural Product from the Pathogenic Bacterium Serratia Marcescens by Caenorhabditis Elegans.” Proceedings of the National Academy of Sciences of the United States of America 104 (7): 2295–2300.
[12] Sengupta, Ankush, Tobias Kruppa, and Hartmut Löwen. 2010. “Chemotactic Predator-Prey Dynamics.” arXiv [q-bio.PE]. arXiv. http://arxiv.org/abs/1009.5521.
[13] Berleman, James E., and John R. Kirby. 2009. “Deciphering the Hunting Strategy of a Bacterial Wolfpack.” FEMS Microbiology Reviews 33 (5): 942–57.
[14] Berleman, James E., and John R. Kirby. 2009. “Deciphering the Hunting Strategy of a Bacterial Wolfpack.” FEMS Microbiology Reviews 33 (5): 942–57.
[15] Reproduced with permission, J. Bacteriol. (2006) 188: 5888–5895, copyright American Society for Microbiology.
[16] Cira NJ, Benusiglio A, Prakash M. Vapour-mediated sensing and motility in two-component droplets. Nature. 2015;519: 446–450. doi:10.1038/nature14272
[17] Cabrera D., Cabrera L., Cabrera, E. A Literature Review of the Universal Patterns and Atomic Elements of Complex Cognition. Journal of Applied Systems Thinking (20) 6. (2020)
.png?width=150&height=150&name=CRL%20GOAT%20Logo%20(4).png)


